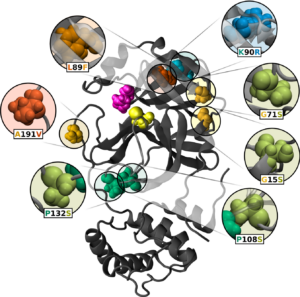
A year's worth of mutations to the SARS-CoV-2 main protease (Mpro) were analyzed by NCASD member Elizabeth M. Diessner, in collaboration with Gemma R. Takahashi from the Martin Lab, to investigate how adaptations of the viral protein may contribute to the spread of the virus behind the Covid-19 pandemic. Published in Biochemistry, this paper details how trends in measurements such as the cohesion and hydrophobicity of the protein reveal vital clues about the structure and dynamics of Mpro, giving insight into the residue interactions that are necessary for the protein to maintain function, as well as those that are more flexible and interchangeable. The significant trends indicate the protein's response to the environment of its human host, and provide information that is valuable for designing inhibitors that will be effective despite continued mutation of the enzyme.